Blogs
It’s all about biovivianite!
Hello there! I am Lordina Eshun, ESR 5, from the University of Manchester, UK. In this blog, I will talk about my secondment at the University of Seville, Spain.
After several postponements due to the Covid-19 pandemic and visa issues, I finally got the opportunity to travel to Seville, the beautiful city and capital of Andalusia on the 29th of October 2021. The initial plan was to stay for 3 months to undertake a secondment at both the University of Seville and Fertiberia, a fertilizer company and a non-academic partner to P-TRAP. This was shortened to 6 weeks obviously due to delays from the Covid-19 pandemic. I was still very happy and excited about this short stay as it gave me the opportunity to test the biovivianite I have produced in the geomicrobiology laboratory through the supervision of Prof. Jon Lloyd, Dr Vicky Coker, and Prof. Samuel Shaw. Here, Biovivianite is vivianite (a phosphate-rich Fe(III) mineral) produced through the microbial transformation of ferrihydrite in the presence of phosphate using Geobacter sulfurreducens (a dissimilatory Fe(III)-reducing bacterium). I had 2 main objectives; one was to determine the effectiveness of biovivianite as an iron source to prevent Fe chlorosis in white lupin, and the second was to test the effectiveness of biovivianite as a phosphorusfertiliser for durum wheat. Using vivianite as a phosphorus fertilizer has not received much attention and most interestingly biovivianite has not been used before. The secondment, therefore, gave me the opportunity to research a novel material (biovivianite) as a fertilizer.
Before the travel!
Prior to travelling to Seville, I performed several lab-based large-scale bioreduction experiments to produce enough biovivianite needed for the plant growth experiments. In the experiments, several parameters were varied and optimised for maximal biovivianite production. Some of these parameters included the amount of phosphate, the starting pH, the rate of Fe(II) production, and the presence or absence of an electron shuttle. When the bioreduction experiment was initiated at a pH below 7, a white precipitate formed (Figure 1B) (identified to be vivianite according to XRD results). However, when the bioreduction experiment was initiated at a pH of 7 or higher, a white precipitate formed together with a brown substance (the precipitate was identified as both vivianite and metavivianite according to XRD results). The brown colour of this product is believed to be the residual ferrihydrite used (Figure 1C). Another set of experiments involved the use of soluble Fe(III) citrate as the starting Fe(III) material (Figure 1A) compared with the insoluble ferrihydrite. Vivianite and green rust were the dominant products formed according to XRD results in these experiments. In total, 3 different types of biovivianite were produced; biovivianite produced with soluble Fe(III) citrate(VivSol) (Figure 1A), biovivianite produced with insoluble ferrihydrite at initial pH of 6.5 (VivInsol6.5) (Figure 1B), and at pH 7.0 (VivInsol7.0) (Figure 1C). After the experiments, the products were washed and suspended in degassed deionised water and kept in sealed serum bottles to minimise oxidation.
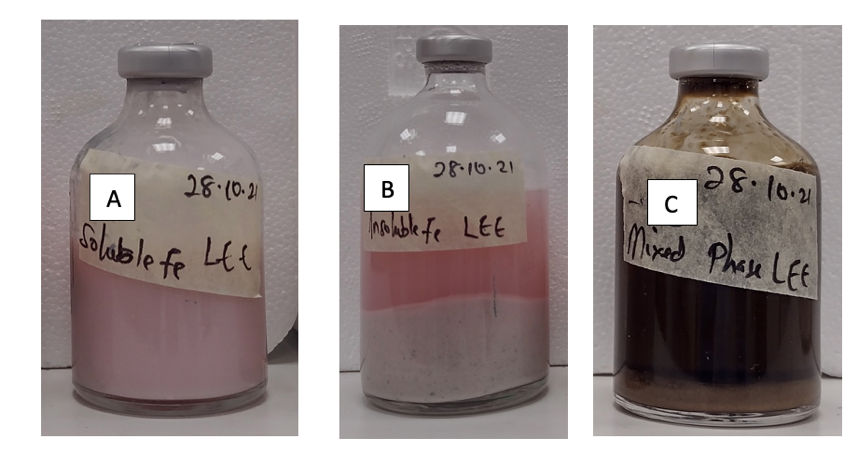
Figure 1: Biovivianite produced using soluble Fe(III) citrate (A), and biovivianite produced using insoluble ferrihydrite at pH 6.5 (B) and 7.0 (C).
Finally in Seville!!!
The day finally came, and I arrived safely in Seville, Spain. I was welcomed into the Agronomy Department by Prof. Antonio Delgado, my supervisor for the secondment. They were very nice to me, they showed me around and gave me a list of some interesting places to visit. With support from the research staff, specifically Ana, Ramiro, Maria, and Isa, I was able to start the growth experiments. I also had the opportunity to visit Fertiberia where I was given a tour of their laboratory, and we had fruitful discussions. One of the challenges I faced during my secondment in Seville was the language barrier. It was very difficult to communicate with people, especially in the shops, on the train and in my accommodation. I used “google translate” in almost all the conversations I had . In as much as this limited the scientific interactions and discussions between my colleagues in Seville and myself, I still believe that the time spent in Seville was one of the major highlights of my PhD journey. During my stay in Seville, I learnt how to perform experiments in a growth chamber, how to measure the chlorophyll content of the leaves using the Minolta SPAD- 502 chlorophyll meter (Minolta Camera Co, Ltd., Osaka, Japan), how to perform acid and alkaline phosphatase activity in the soil, Olsen P measurements and how to quantify DTPA extracted Fe from the soil. In one of my lab experiments, I felt light-headed and almost fell if not for the help of one of the colleagues who saw me and took me to the hospital. Ironically, I was anaemic although I was working with iron .
Although the workload for the growth experiments demanded that I work at the weekends, I also got some time to explore the beautiful city of Seville, enjoyed the nice weather and met Tolulope (ESR 8) for the first time after several online meetings.
Is Biovivianite an effective Fe and P source???
The results from the experiments were very interesting and positive. We found out that not only was biovivianite effective as a Fe source for lupin, but it was also an effective phosphorus fertilizer for the wheat plant. Specifically, biovivianite produced at initial pH of 7.0 (VivInsol7.0 Figure 1C) was effective as the Fe fertilizer whereas the biovivianite produced using soluble Fe(III) citrate (VivSol Figure 1A) was a better phosphorus source to the wheat plants. We observed from the results that, the solubility of the Fe(III) starting material, the starting pH used during bioreduction, the mineral constituents and the particle sizes of the fertilizer products have a major influence on its uptake as a plant fertilizer.
I would like to thank my supervisors from the University of Manchester (specifically Prof. Jonathan Lloyd), my supervisor from the University of Seville (Prof. Antonio Delgado), the research staff at the Agronomy Department (Ana Maria García-López, Ramiro Racena, Maria, and Isa and all the other colleagues in the department) for their support and guidance during this period.

