Blogs
New year – new challenges!
This week we started with the first part of our 2nd P-TRAP school. Unfortunately back online, although most of us were secretly hoping for loads of Belgian waffles!
 The course aims to give an introduction on speciation codes and to get some hands-on about Fe-P interactions. Those speciation codes help to solve biogeochemical equations such as given in redox reactions, solubilities sorption or interactions between inorganic and organic materials such as Dissolved Organic Matter (DOM).
The course aims to give an introduction on speciation codes and to get some hands-on about Fe-P interactions. Those speciation codes help to solve biogeochemical equations such as given in redox reactions, solubilities sorption or interactions between inorganic and organic materials such as Dissolved Organic Matter (DOM).
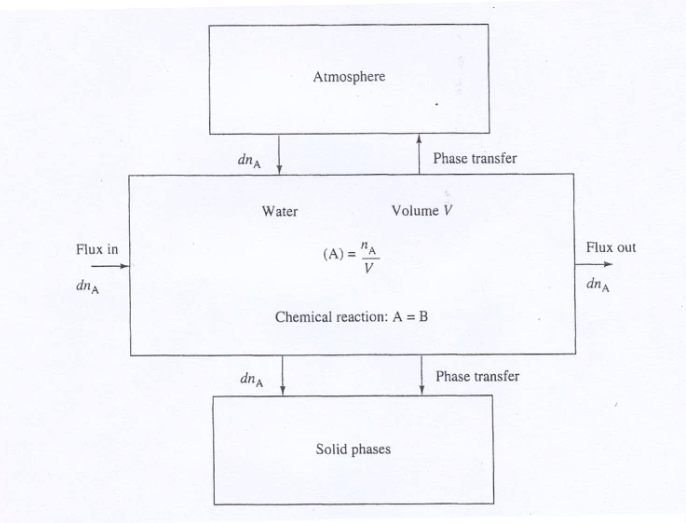
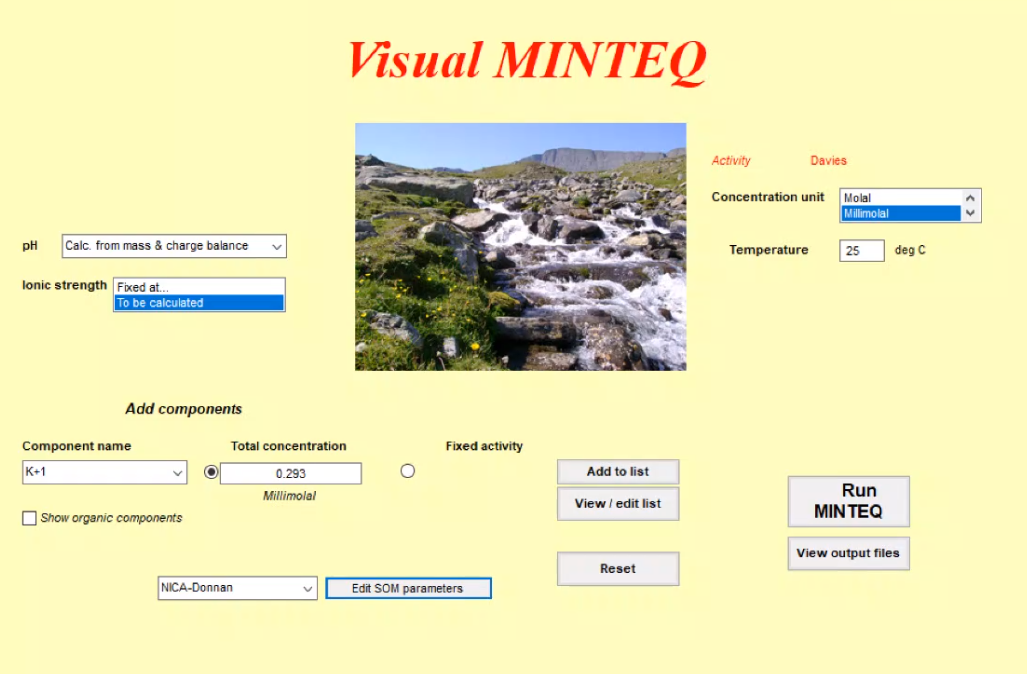
We decided to use Visual MINTEQ, the oldest of those model applications. It is a box model, calculations are based on chemical equilibriums but give no fluxes between the boxes. There are also several others on the market such as GEOCHEM or PHREEQC, but Visual MINTEQ still uses the original codes, and although it looks a bit old fashioned, it has a good data base and visualizations. Other advantages are that it also includes toxicity, which is a not that common tool, and that it is freeware. So, all students can easily work with it and benefit for their projects.
After half an hour of introduction about the chemistry behind it and another half an hour how Visual MINTEQ is working, the ESRs got in touch with the real model. In advance they received lots of calculation exercise challenges, which they had to solve now individually and as a team.
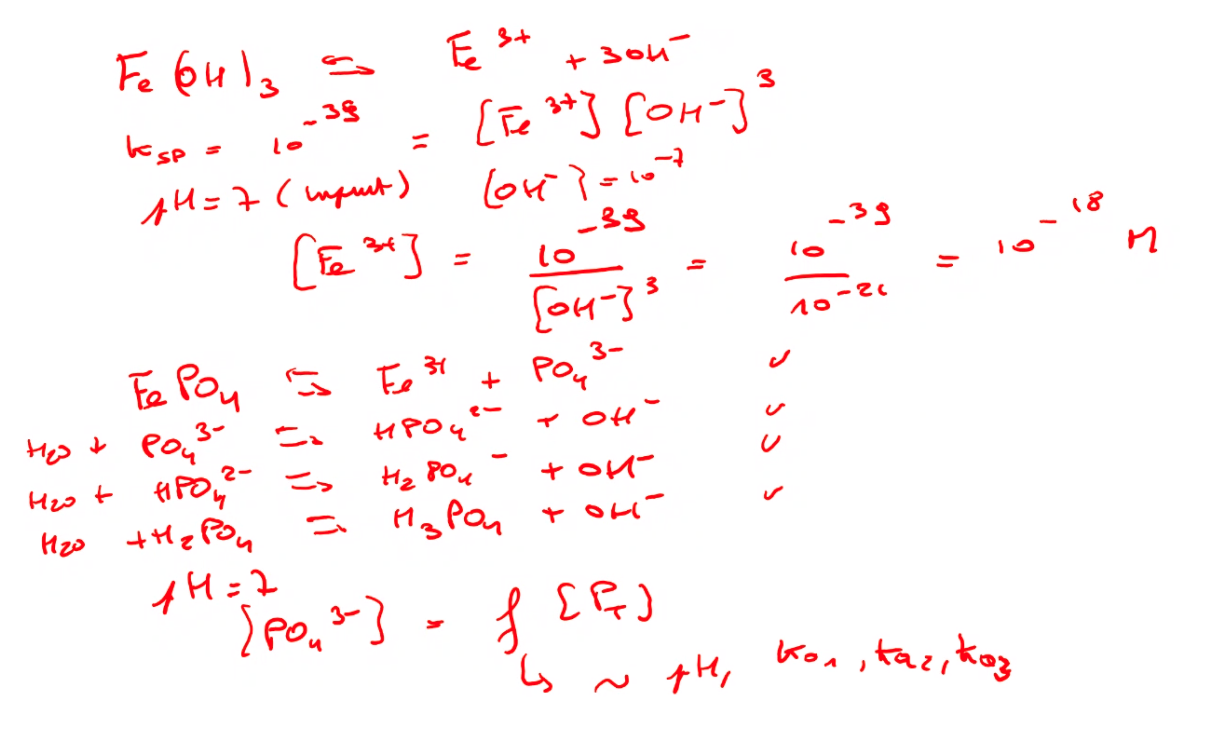 |
 |
 |
|
Some basics! |
Boxes of a chemical model |
The Visual MINTEQ surface |
They learned about the importance of the right parameters and their units to solve the equations, not only to fill in in the model, but also which are need to be measured in the field. Such knowledge is indispensable when planning your own campaigns. One missing parameter, e.g. a relatively simple one like the pH value, can jeopardize all your measurement interpretations! They also learned to rank the parameters. Pressure for example is in our case negligible, but temperature is not, as it affects the solubility of gas in water much more than pressure. This means, that if you have to choose between buying a pressure sensor or spend your money instead on a very sensitive temperature sensor, it is clear what to do.
This leads to another import topic in the scientific world: errors. Not only how to avoid them practically, but also the need of calculating them and to check how your results are impacted by e.g. the sampling method, the chosen measurement conditions, the calculations itself, and so on. Similar to the ranking of the parameters it is important to realise and determine the impact of different errors on your results. This is done by calculating the error propagation. Knowing how much a result is influenced by a specific handling, treatment and calculation helps to improve the steps from the measurements to the final data. And to decide how much decimal places you need to use! The Fe activity is changing by a factor of 1000 if you change the pH by only one unit!
Next week it is time for a completely different topic. The ESRs will be introduced to our transferable skills training: How to communicate your science out of the ivory tower?
To be continued….

